Our Company
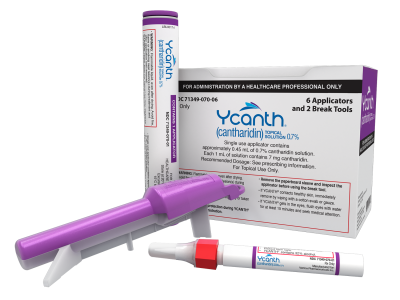
Verrica Pharmaceuticals Inc. is a dermatology therapeutics company developing medications for skin diseases requiring medical interventions. We believe there’s a better way to safely and effectively bring relief to patients, deliver peace of mind to parents and caregivers, and advance accessible therapies to prescribers and payers. We are focused on changing the narrative around skin diseases and making treatment a more efficient process for both physicians and patients. On July 21, 2023, Verrica’s lead product, YCANTH™ (cantharidin) topical solution 0.7%, became the first treatment approved by the FDA to treat adult and pediatric patients as young as two years of age with molluscum contagiosum, a highly contagious, viral skin disease infection affecting approximately 6 million people in the United States, primarily children.
Please see Important Safety Information and full Prescribing Information for YCANTH.
Verrica is in process of developing VP-102, an investigational product, for the treatment of common warts and external genital warts, two of the largest unmet needs in medical dermatology. Verrica is developing VP-103, its second investigational cantharidin-based product, for the treatment of plantar warts.
Verrica has also entered a worldwide license agreement with Lytix Biopharma AS (“Lytix”) to develop and commercialize VP-315 for dermatologic oncology conditions. Verrica intends to focus initially on basal cell and squamous cell carcinomas as the lead indication for development.