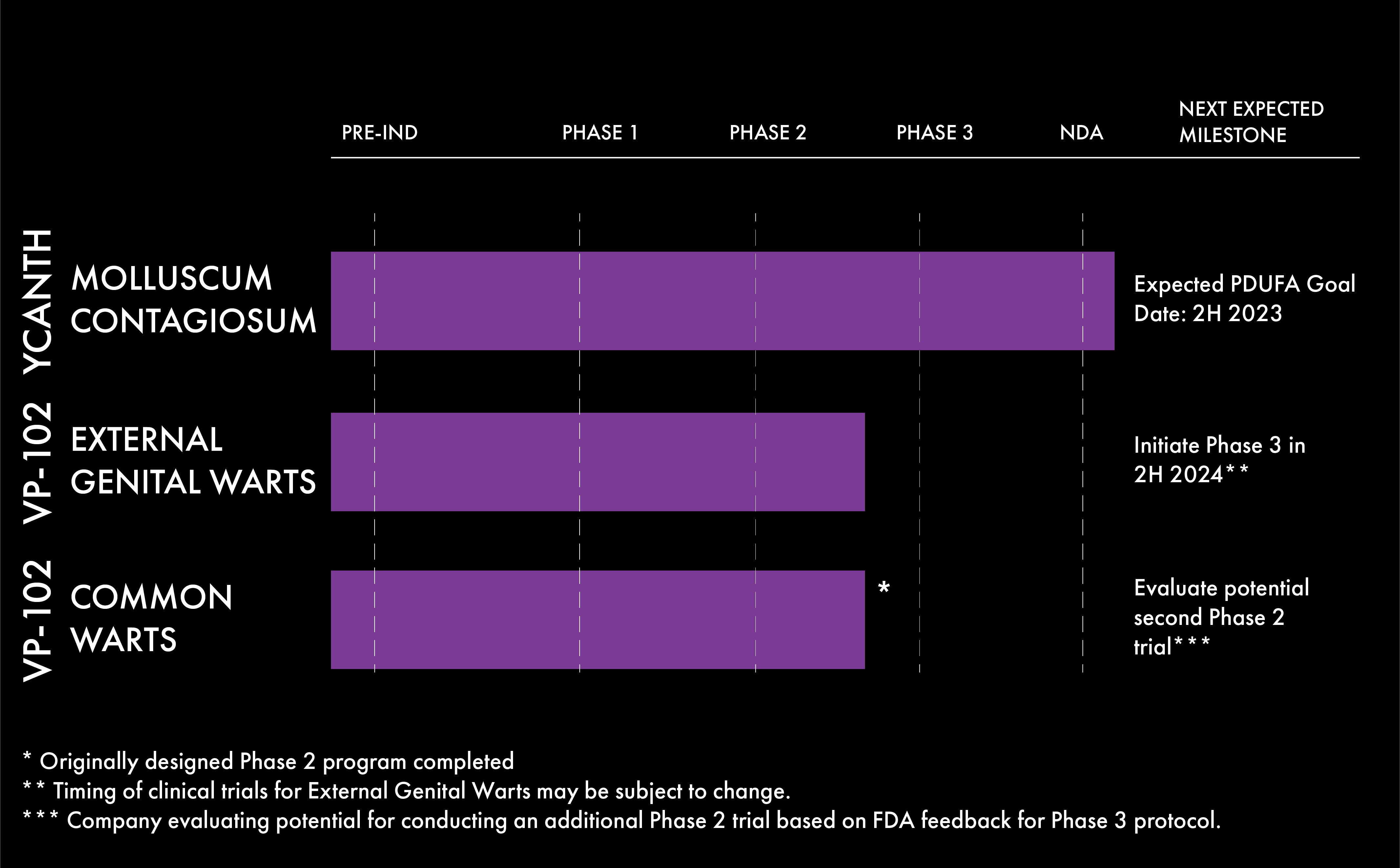
Pipeline VP-102
Verricaʼs lead product candidate, VP-102, is a proprietary drug-device combination that contains a GMP-controlled formulation of cantharidin (0.7% w/v) delivered via a single-use applicator that allows for precise topical dosing and targeted administration. We are developing VP-102 for the treatment of molluscum contagiosum (molluscum), common warts and external genital warts, three of the most common viral skin diseases in medical dermatology.
VP-102 has the potential to be the first product approved by the FDA to treat molluscum ― a common, highly contagious skin disease that affects an estimated six million people in the United States, primarily children. If approved, VP-102 will be marketed in the United States under the conditionally accepted brand name, YCANTH™.
CONTAGIOSUM
GENITAL WARTS
WARTS
in 2H 2024**
** Timing of trial may be subject to change.
*** Company evaluating potential for conducting an additional Phase 2 trial based on FDA feedback for Phase 3 protocol.
VP-102 is currently under U.S. Food and Drug Administration (FDA) review and could potentially be the first product approved by the FDA to treat molluscum contagiosum (molluscum)― a common, highly contagious skin disease that affects an estimated six million people in the United States, primarily children. If approved, VP-102 will be marketed in the United States under the conditionally accepted brand name, YCANTH™.
Successful Phase 3 Results in Molluscum
Verricaʼs Phase 3 program with VP-102 for the treatment of molluscum included the first successful pivotal studies to date for these patients.
Verrica conducted two identical Phase 3 randomized, double-blind clinical trials (CAMP-1 and CAMP-2) to evaluate the safety and efficacy of VP-102 compared to vehicle in subjects two years of age and older. The studies enrolled 528 subjects in total and were conducted at 31 centers in the United States. In both trials, a clinically and statistically significant percentage of subjects treated with VP-102 achieved complete clearance of all treatable molluscum lesions, the primary endpoint, compared to vehicle.
Molluscum Contagiosum
There are currently no FDA-approved treatments for molluscum contagiosum (molluscum), a highly contagious viral skin disease that affects approximately six million people — primarily children — in the United States. Molluscum is caused by a pox virus that produces distinctive raised, skin-toned-to-pink-colored lesions that can cause pain, inflammation, itching and bacterial infection. It is easily transmitted through direct skin-to-skin contact or through fomites (objects that carry the disease like toys, towels or wet surfaces) and can spread to other parts of the body or to other people, including siblings. The lesions can be found on most areas of the body and may carry substantial social stigma. Without treatment, molluscum can last for an average of 13 months, and in some cases, up to several years.
In Phase 3 studies evaluating VP-102 for the treatment of molluscum, a clinically and statistically significant percentage of patients treated with VP-102 achieved complete clearance of all new and baseline molluscum lesions compared to vehicle.
Common Warts
Common warts (verruca vulgaris) are contagious skin growths caused by a human papilloma virus (HPV). They represent one of the largest unmet needs in dermatology, with approximately 22 million cases in the United States. There are no FDA-approved treatments for common warts, which can be difficult to treat and negatively affect quality of life.
Verrica has announced positive top-line results from its COVE-1 Phase 2 open-label clinical study of VP-102 for the treatment of common warts.
External Genital Warts
External genital warts are a viral skin disease caused by human papilloma virus, or HPV, which forms lesions on the surface of the skin on or near the genitals. HPV is the most common sexually transmitted infection in the United States. Verrica is currently conducting a Phase 2 study of VP-102 for the treatment of external genital warts.